Theme:
Biopharma 2021
Bio pharmaceutics and Biologic Drugs
Aug 2-3, 2021|Webinar
Conference Series LLC Ltd welcomes you to attend the 2nd International Conference and Expo on Bio pharmaceutics and Biologic Drugs to be held on Aug 2-3, 2021. The theme for the conference this year is Novel Methodologies in Pharmaceutical and Biologic Drugs
Details of Biopharma 2021 Webinar
|
Conference Name |
Place |
Date |
|
Biopharma 2021 |
Webinar |
Aug 2-3, 2021 |
About Webinar
Conference Series Ltd extends its welcome to 2nd International Conference and Expo on Bio pharmaceutics and Biologic Drugs on Aug 2nd and 3rd 2021 (Webinar). Conference Series Ltd through its Open Access Initiative is committed to make genuine and reliable contributions to the scientific community.
Conference Series Ltd Organizes 1000+ Conferences Every Year across USA, Europe & Asia with support from 1000 more scientific societies and Publishes 700+ Open access journals which contains over 100000 eminent personalities, reputed scientists as editorial board members.
This conference provides a forum for bio pharmaceutics students, instructors, deans, researchers, and leaders to discuss issues affecting biologic pharmaceuticals. A mixture of oral and poster presentations will provide attendees with opportunity to learn about drug and design research. Meet and network with a diverse group of nurses, including students, deans, educators, and researchers.
Target Audience
- Quality control specialist
- Graduates and post graduates in industrial pharmacy
- Medical Devices Manufacturing Companies, CRO
- Data Management Companies.
- Pharmaceutical legislators and regulators
- Researchers
- Directors, CEO’s of Organizations
- Scholars from Pharmaceutical backgrounds
- Drug Delivery Technology Manufacturers
- Business Development Managers
- Distributors and Suppliers of Drug Delivery Technologies
- Students, Professors, Researchers, and Faculty of Pharmaceutical Sciences from Universities and Medical Colleges
- Researchers from Pharmaceutical Companies, Pharmacy Associations and Societies
- Health professionals Pharmacists
- Business development professionals, Consultants and Pharma service providers
Motives to attend:
- Keynote presentation along with interactions to galvanize the scientific community.
- Workshop and symposiums to reach the largest assemblage of participants from the Pharma community.
- A wide track of exhibitors to showcase the new and emerging technologies.
- Platform to global investment community to connect with stakeholders in Pharma sector.
- Young Scientist/ Investigators Award geared towards best budding young research.
- Links to the political marketing resources in order to expand your business and research network.
- Triumph of Awards, Certificates recognizes your commitment to your profession to encourage the nascent research.
Track 1: Globalized Pharma Sector
The globalised pharmaceutical industry is based on gathering information on changing competition structures and greater competitiveness, a lack of brand new drugs despite higher R&D expenditure, growing importance of regulatory issues (registrations, intellectual property rights, litigations), and the rapid consolidation and concentration of the global pharmaceutical industry.
Related Societies:
Track 2: Drug Discovery
Drug discovery is the procedure for discovering novel candidate pharmaceuticals in the domains of medicine, biotechnology, and pharmacology. Modern drug development entails identifying screening hits, medicinal chemistry, and optimization of those hits to improve affinity, selectivity (to lower the risk of adverse effects), efficacy or potency, and metabolic stability (to enhance the medication's shelf life).
Related Societies:
Track 3: Drug Development
Once a lead molecule has been identified through the drug discovery process, drug development focuses on bringing a new pharmaceutical medicine to market. It may include pre-clinical research on microbes and animals, as well as submitting for regulatory status, such as an investigational new drug application with the US Food and Drug Administration to begin human clinical trials.
Related Societies:
Track 4: Pharmaceutical Engineering
Pharmaceutical engineering is concerned with pharmaceutical science and technology, which includes the creation and manufacture of pharmaceutical products, procedures, and components (i.e. drugs & biologics). Pharmaceutical product development entails a wide range of specialties (e.g. medicinal chemists, analytical chemists, clinicians/pharmacologists, pharmacists, chemical engineers, etc.).
Related Societies:
Track 5: Advances in Pharmaceutical Packaging
During storage, transit, exhibition, and until a product is safely consumed, packaging plays a crucial role in providing protection, presentation, convenience, identity information, and compliance. Packaging can be thought of as a mechanism for getting a product from the manufacturer to the consumer in a safe manner. The focus of the subject is mostly on contemporary advancements in packaging technology and packaging design.
Related Societies:
Track 6: Drug Controlisation
Medication development is the process of getting a new drug molecule into clinical use. This includes all stages of the drug development process, from fundamental research through commercialization. The clinical aspects of this process are referred to as development, while the nonclinical research components are referred to as discovery. A promising chemical for development has been discovered by researchers.
Related Societies:
Track 7: Drug Tolerance
Drug tolerance is a sign of drug use; however it isn't always linked to drug addiction or dependence. Tolerance development is a reversible process that can be influenced by both physiological and psychological variables. Drug tolerance should not be confused with medicine tolerability, which refers to the degree to which a patient can tolerate a drug's side effects.
Related Societies:
Track 8: Digital Pharma
Some people believe that digital skills are crucial not only for pharmaceutical companies' ability to enhance how they roll out new products, but also for improving the pharmaceutical industry's contribution to health care by enabling it to provide novel services that improve patient outcomes. To improve Pharma's perceived value in the health-care system, the industry is working to meet the demands of all stakeholders, including health-care providers, patients, and payers. Meanwhile, the pharmaceutical industry's mentality is evolving from vertically organised departments to cross-functional collaboration.
Related Societies:
Track 9: Regulatory Requirements for Pharmaceuticals
There are numerous reasons for expanding merchandise development outside of mature, developed economies (e.g., the EU and hence the US), and the majority of them are based on the growing markets' large population and market potential. The FDA's clinical investigation and pharmaceutical development advice is becoming increasingly multinational in its approach to emerging markets.
Related Societies:
Track 10: Clinical Trials and Regulatory Affairs in Pharmacy
Clinical research work is directly associated to patient outcomes, such as in clinical trials. People within the clinical research field feel that their jobs are extremely impactful, as they are at the very forefront of wherever science becomes medicine that can improve patients' lives. Regulatory affairs (RA) scientists are involved overseeing the process of getting a drug or product through the FDA review and approval process and onto the market. Since every step in the process of product development is highly regulated, those in RA work at every step to move a drug from research and development through FDA approval.
Related Societies:
Tracks 11: Smart Drug Delivery Systems
Many clinically valuable medications have been hampered by toxicity, and Smart Drug Delivery Systems (SDDS) have emerged as a panacea. The discovery of Amphotericin B as a liposome has sparked excitement and prompted massive efforts in the field of SDDS. The concept of toxicity manipulation has spawned a slew of new techniques, including targeted and physico-chemical techniques. Targeting liposomes, drug-loaded biodegradable microspheres, stimuli-responsive drug polymer conjugates, smart hydrogels, polymeric micellar particles, intelligent lipoprotein carriers, and nano carriers are among the SDDS products currently available.
Related Societies:
Track 12: Peptides and Protein Drug Delivery
Novel drug delivery systems include protein and peptide drug delivery systems. The most abundant components of living cells are proteins and peptides. Enzymes, hormones, structural elements, and immunoglobulin all have functions. Peptide bonds connect the twenty distinct naturally occurring amino acids, forming polymers known as peptides and proteins. Because of their unfavourable physicochemical features, such as enzymatic breakdown, poor membrane permeability, and big size, designing and producing a polypeptide medication delivery across the gastro intestinal system has been a persistent issue.
Related Societies:
Track 13: Drug Safety and Pharmacovigilance
In the world of medicine, drug safety is known as medication safety. It has to do with the antagonistic effects of pharmaceutical items, as well as a variety of other logical views, such as medication responses, solution nature, and prescription error in medication use, medication viability, and phoney pharmaceuticals. Pharmacovigilance supports overall health programmes by providing reliable data for the effective assessment of a solution's risk-benefit profile. It also contributes to the evaluation of formal, uses, symptoms, damage, viability, and danger of pharmaceuticals, allowing for the safe, sound, and more viable usage of various treatments. Pharm students receive advanced training, comprehension, and clinical preparation.
Related Societies:
Track 14: Pharmaceutical Biotechnology
Pharmaceutical biotechnology has become one of the most important fields in medication development. Today, the shape and perspective of pharmaceutical elements and issues have entirely altered, and the word pharma can be regarded as a term for integrated life science techniques spanning from genetics to molecular biology to diagnostics, all with the shared objective of giving the best possible outcomes.
Related Societies:
Track 15: Pharmacogenetics and Genomics
Pharmacogenetics is the science that helps us understand the role that a person's hereditary make-up plays in how well a prescription works, as well as what symptoms are likely to occur, allowing us to better separate the hereditary causes of illnesses and find novel treatments. Pharmacogenetics refers to hereditary differences in metabolic pathways that might influence individual medication reactions, both in terms of therapeutic and antagonistic effects.
Pharmacogenomics is a rapidly growing field with important implications in personalised patient care. Its recommendations can help with concerns like medication safety and development. Pharmacogenomics consolidates customary pharmaceutical sciences, for example, natural chemistry with explained colleague of qualities, proteins, and single nucleotide polymorphisms.
Related Societies:
Global Business & Research Value on Pharmaceutical
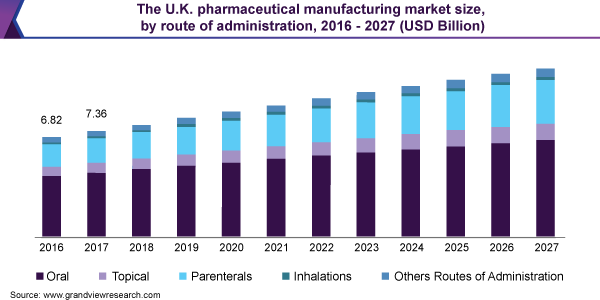
Since 2015, the worldwide medicines industry has grown at a compound annual growth rate (CAGR) of 6.7 percent, reaching almost $1,217.1 billion in 2019. The market is predicted to fall by 0.6 percent from $1,217.1 billion in 2019 to $1,209.6 billion in 2020. The reduction is primarily due to various countries' forced lockdown and social distancing norms, as well as a global economic recession. From 2021 to 2023, the market is predicted to recover and grow at an annual rate of 8.5 percent, reaching $1,738.2 billion. In 2025, the market is estimated to reach $2,050.9 billion, and in 2030, $3,206.3 billion. Technological advancements, changes in lifestyles, new approaches for drug development, a big pool of undiagnosed people, and an increase in pharmaceutical drug usage owing to the COVID-19 pandemic will all contribute to the rise in pharmaceutical drug usage.
The "Medical Gases Market" 2021 by Types (Medical Oxygen, Medical Nitrous Oxide, Medical Air, Medical Helium, Others), Application (Hospitals (Labs and Clinics), Universities/Research Institutions, Pharmaceutical and Biotechnology Industries) and Region - Global Forecast to 2025", according to 360 Research Reports, is expected to grow at a significant rate during the forecast period.
COVID-19 has the potential to have three major effects on the global economy: directly impacting production and demand, causing supply chain and market disruption, and having a financial impact on businesses and financial markets.
Future Scope
The health of the future that we foresee in 2040 will be nothing like what we have now. We may reasonably expect digital transformation—enabled by fundamentally interoperable data, AI, and open, secure platforms—to drive much of this shift, based on upcoming technology. We believe that, unlike today, care will be organised around the consumer rather than the institutions that drive our economy. Streams of health data, together with data from a range of other relevant sources, will most likely merge by 2040 (and possibly much before) to generate a complex and highly tailored picture of each consumer's well-being. Many digital health companies are already incorporating always-on biosensors and software into data-generating, data-gathering, and data-sharing devices.
The age of blockbuster pharmaceuticals that treat big populations is expected to fade in the future of health. Rather than treating symptoms, the biopharma industry is on the cusp of a new era in which hypertailored treatments are produced to cure or prevent disease. Personalized medications based on a diverse set of a patient's features, including genomes, metabolome, microbiome, and other clinical information, may be created or compounded just in time by additive manufacturing twenty years from now, rather than picking up a prescription at the pharmacy
Conference Highlights
- Drug Discovery
- Advances in Pharmaceutical Packaging
- Globalized Pharma Sector
- Digital Pharma
- Regulatory Requirements for Pharmaceuticals
- Clinical Trials and Regulatory Affairs in Pharmacy
- Smart Drug Delivery Systems
- Peptides and Protein Drug Delivery
- Drug Safety and Pharmacovigilance
- Pharmaceutical Biotechnology
- Pharmacogenetics and Genomics
- Drug Development
- Pharmaceutical Engineering
- Drug Controlisation
- Drug Tolerance
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | August 02-03, 2021 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | |||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Journal of Pharmaceutica Analytica Acta
- Journal of Clinical Pharmacology & Biopharmaceutics
- Journal of Pharmaceutical Regulatory Affairs
Abstracts will be provided with Digital Object Identifier by


