Theme: Novel Strategies and Advancements in Biopharmaceutics
Euro Biopharma 2018
The Euro Biopharma 2018 offers a best platform with its well organized scientific program to the audience which includes interactive panel discussions, keynote lectures, plenary talks and poster sessions on the topics cell therapy, biotechnology,therapeutic biological products,gene therapy, Biosimilars, BABE, clinical trials, Drug discovery, current issues in Bioequivalence, Pharmaceutical Innovation in the 21st Century, new scientific approaches to international regulatory standards and Biophamaceutical companies &market analysis . The conference invites delegates from Biopharma laboratories, Pharmacists, Academicians, Clinicians, Researchers, Health care professionals, students, business delegates and Young researchers across the globe providing a better podium, interconnecting the latest research, technological developments in the arena as well as therapeutic aspects.
Conference Series llc LTD organizes a conference series of 1000+ Global Events inclusive of 600+ Conferences, 500+ Upcoming and Previous Symposiums and Workshops in Europe & Asia, USA with support from 1000 more scientific Societies and publishes 700+ Open Access Journals which contains over 50000 eminent personalities, reputed scientists as editorial board members.
Why to Attend:
With all the scientific people over the world focused on learning about advancements in Pharma community. This is a best globalized opportunity to reach the largest assemblage of participants. We anticipate participants, renowned speakers and eminent delegates across the globe attending the conference to share their valuable presentation and galvanize the scientific community. Euro Biopharma2018 is to conduct the presentations, distribute information, meet with potential scientists, make a splash with new developments, and receive fame and recognition. Our services have always met with great achievement in Business Conferencing, the most recent and advanced techniques, developments, and the newest updates are the prominent features of the conference.
Conference highlights & tracks:
Cell Therapy, Therapeutic Biological Products, Gene Therapy, Biosimilars, Bioavailability/Bioequivalence, Clinical Trails, Drug Discovery, Biotechnology, Drug Delivery System, Drug Disposition & Pharmacokinetics, Drug Metabolism, Biopharmaceutical Companies & Market Analysis, Pharmaceutical Regulatory Affairs And Certifications
Conference Series llc LTD invites all the participants from all over the world to attend European Biopharma Congress during September 18-19, 2018 in Amsterdam, Netherlands. Which includes prompt Keynote presentations, Oral talks, Poster presentations and Exhibitions.
The Euro Biopharma 2018 offers a best platform with its well organized scientific program to the audience which includes interactive panel discussions, keynote lectures, plenary talks and poster sessions on the topics Cell Therapy, Biopharmaceutics, Drug delivery, Biotechnology, Gene Therapy, Therapeutic Biological Products, BABE, Biosimilars, Clinical Trails, Drug Discovery, current issues in Bioequivalence, Pharmaceutical Innovation in the 21st Century, new scientific approaches to international regulatory standards. Biopharmaceutical Companies & Market Analysis, The conference invites delegates from Biopharma laboratories, Pharmacists, Academicians, Clinicians, Researchers, Health care professionals, students, business delegates and Young researchers across the globe providing a better podium, interconnecting the latest research, technological developments in the arena as well as therapeutic aspects.
Track 1: Biopharmaceutics: Drug Discovery and Development
The field of new drug development has moved its focus from small-molecule compounds to large-molecule proteins and other biopharmaceuticals. These biopharmaceuticals have caused an archetype shift in disease cure and has led to an enhancement in the quality of life of patients with various diseases such as autoimmune diseases, refractory cancers biopharmaceuticals represent 7.5% of all drugs in the market and account for ~10% of the total expenses for marketed drugs. The usage of biopharmaceuticals are increasing at the rate >20% per year. They are already being used74% more than chemically derived pharmaceuticals, in life-saving or end-stage applications. Moreover, biopharmaceuticals covers > 30% of all pipeline research programs going-on at present which embraces all recombinant proteins, monoclonal antibodies, cytokines, peptides, vaccines, blood/plasma-derived products, non-recombinant culture-derived proteins, nucleic acid-based products (DNA, RNA or antisense oligonucleotides used for curative or in vivo diagnostic purposes), cells and tissues cultures. This view is possibly best understood by the public and mostly used by the people in the biopharmaceutical industry.
Related Conferences: Pharmaceutical Conferences | Biopharmaceutical Events | Pharma Conferences | Pharmaceutical Meetings | Biopharma Congress
8th Global Summit on Microbiology & Infectious Diseases, February 22-23, 2018 Paris, France; 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology, April 09-11, 2018 Amsterdam, Netherlands; 4th International Conference on Polymer Chemistry, June 25-27, 2018 Stockholm, Sweden; 11th World Microbiology & Immunology Congress, June 28-29, 2018 Amsterdam, Netherlands; 10th International Conference on Chemistry Education, June 21-22, 2018 Oslo, Norway;12th World Congress on Biotechnology and Microbiology, June 28-29, 2018 Amsterdam, Netherlands;14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, 10th World Congress on Neuropharmacology, August 28-30, 2018 Paris, France; Sweden; 8th World Congress on Chromatography, September 13-14, 2018 Prague, Czech Republic; 9th World Congress on Green Chemistry and Technology, September 17-19, 2018 Amsterdam, Netherlands; 17th Annual Congress on Pharmaceutics & Drug Delivery Systems, September 20-22, 2018 Prague, Czech Republic
Related Societies:
Association of the British Pharmaceutical Industry (ABPI), International Association for Pharmaceutical Technology(APV), National Pharmacy Association (NPA), Dutch Society for Clinical Pharmacology & Biopharmacy, European Association of Clinical Pharmacology and Therapeutics (EACPT), The International Society for Biopharmaceutical Statistics (ISBS), Chinese American BioPharmaceutical Society
Track 2:Therapeutic Biological Products
Products that are obtained from specific organs or tissues said to correspond with the unhealthy organs or tissues of the recipient. Proponents claim that the recipient's body automatically transports the injected cells to the target organs, where they supposedly strengthen them and regenerate their structure. The organs and glands used in cell treatment include brain, pituitary, thyroid, adrenals, thymus, liver, kidney, pancreas, spleen, heart, ovary, testis, and parotid. Several different types of cell or cell extract can be given simultaneously - some practitioners routinely give up to 20 or more at once.
Related Conferences: Pharmaceutical Conferences | Biopharmaceutical Events | Pharma Conferences | Pharmaceutical Meetings | Biopharma Congress
8th Global Summit on Microbiology & Infectious Diseases, February 22-23, 2018 Paris, France; 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology, April 09-11, 2018 Amsterdam, Netherlands; 4th International Conference on Polymer Chemistry, June 25-27, 2018 Stockholm, Sweden; 11th World Microbiology & Immunology Congress, June 28-29, 2018 Amsterdam, Netherlands; 10th International Conference on Chemistry Education, June 21-22, 2018 Oslo, Norway;12th World Congress on Biotechnology and Microbiology, June 28-29, 2018 Amsterdam, Netherlands;14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, 10th World Congress on Neuropharmacology, August 28-30, 2018 Paris, France; Sweden; 8th World Congress on Chromatography, September 13-14, 2018 Prague, Czech Republic; 9th World Congress on Green Chemistry and Technology, September 17-19, 2018 Amsterdam, Netherlands; 17th Annual Congress on Pharmaceutics & Drug Delivery Systems, September 20-22, 2018 Prague, Czech Republic
Related Associations or Societies: Generic Pharmaceutical Association | US Food and Drug Administration( FDA) | European Generic medicines Association | Therapeutics Goods Administration (TGA) | European Economic Area | Canadian Generic Pharmaceutical Association (CGPA) | Bioequivalence and Bioavailability forum | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry (AACC) | American Association of Pharmaceutical Scientists (AAPS) | Clinical Trials Information from National Institutes for Health (NIH) | National Institute of Standards and Technology (NIST)
Track 3: Cellular and Gene Therapy
Cell therapy as performed by alternative medicine practitioners is very different from the controlled research done by conventional stem cell medical researchers. Alternative practitioners refer to their form of cell therapy by several other different names including Immunotherapy, Allogenic Cell Therapy and fresh Neural Stem cell therapy. Proponents of cell therapy claim that it has been used successfully to rebuild damaged cartilage in joints, repair spinal cord injuries, strengthen a weakened immune system, treat autoimmune diseases such as AIDS, and help patients with neurological disorders such as Alzheimer’s disease, Parkinson's disease and epilepsy.
Gene therapy and cell therapy are overlapping fields of biomedical research with the goals of repairing the direct cause of genetic diseases in the DNA or cellular population, respectively. The development of suitable gene therapy treatments for many genetic diseases and some acquired diseases has encountered many challenges and uncovered new insights into gene interactions and regulation. Further development often involves uncovering basic scientific knowledge of the affected tissues, cells, and genes, as well as redesigning vectors, formulations, and regulatory cassettes.
Related Conferences: Pharmaceutical Conferences | Biopharmaceutical Events | Pharma Conferences | Pharmaceutical Meetings | Biopharma Congress
8th Global Summit on Microbiology & Infectious Diseases, February 22-23, 2018 Paris, France; 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology, April 09-11, 2018 Amsterdam, Netherlands; 4th International Conference on Polymer Chemistry, June 25-27, 2018 Stockholm, Sweden; 11th World Microbiology & Immunology Congress, June 28-29, 2018 Amsterdam, Netherlands; 10th International Conference on Chemistry Education, June 21-22, 2018 Oslo, Norway;12th World Congress on Biotechnology and Microbiology, June 28-29, 2018 Amsterdam, Netherlands;14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, 10th World Congress on Neuropharmacology, August 28-30, 2018 Paris, France; Sweden; 8th World Congress on Chromatography, September 13-14, 2018 Prague, Czech Republic; 9th World Congress on Green Chemistry and Technology, September 17-19, 2018 Amsterdam, Netherlands; 17th Annual Congress on Pharmaceutics & Drug Delivery Systems, September 20-22, 2018 Prague, Czech Republic
Related Societies:
Association of the British Pharmaceutical Industry (ABPI), International Association for Pharmaceutical Technology(APV), National Pharmacy Association (NPA), Dutch Society for Clinical Pharmacology & Biopharmacy, European Association of Clinical Pharmacology and Therapeutics (EACPT), The International Society for Biopharmaceutical Statistics (ISBS), Chinese American BioPharmaceutical Society
Track 4: Biologics and Biosimilars
The development of biologics calls for overcoming lot many challenges. With initial steps of concepts of biologics, their considerations, essentials for early clinical developments it is very much needed that proper scientific and strategic approaches are taken for the successful development of follow-on-biologics. Moreover, the need for overcoming the challenges continues in the late clinical steps, drug safety factors and labelling requirements. Also it is much required now to develop a drug product in accordance to quality by design (QBD). This Euro Biopharma 2017 conference will look at the multiple facets of current challenges in biosimilar development. This conference will focus on multiple aspects of biosimilar product development to successfully deliver safe, potential and efficacious biologic products to the market.
Related Conferences: Pharmaceutical Conferences | Biopharmaceutical Events | Pharma Conferences | Pharmaceutical Meetings | Biopharma Congress
8th Global Summit on Microbiology & Infectious Diseases, February 22-23, 2018 Paris, France; 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology, April 09-11, 2018 Amsterdam, Netherlands; 4th International Conference on Polymer Chemistry, June 25-27, 2018 Stockholm, Sweden; 11th World Microbiology & Immunology Congress, June 28-29, 2018 Amsterdam, Netherlands; 10th International Conference on Chemistry Education, June 21-22, 2018 Oslo, Norway;12th World Congress on Biotechnology and Microbiology, June 28-29, 2018 Amsterdam, Netherlands;14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, 10th World Congress on Neuropharmacology, August 28-30, 2018 Paris, France; Sweden; 8th World Congress on Chromatography, September 13-14, 2018 Prague, Czech Republic; 9th World Congress on Green Chemistry and Technology, September 17-19, 2018 Amsterdam, Netherlands; 17th Annual Congress on Pharmaceutics & Drug Delivery Systems, September 20-22, 2018 Prague, Czech Republic
Related Associations or Societies: Generic Pharmaceutical Association | US Food and Drug Administration( FDA) | European Generic medicines Association | Therapeutics Goods Administration (TGA) | European Economic Area | Canadian Generic Pharmaceutical Association (CGPA) | Bioequivalence and Bioavailability forum | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry (AACC) | American Association of Pharmaceutical Scientists (AAPS) | Clinical Trials Information from National Institutes for Health (NIH) | National Institute of Standards and Technology (NIST)
Track 5: Bioavailability & Bioequivalence studies of Biopharmaceuticals
The Bioavailability Bioequivalence Research Center aims to become a regional center of excellence for assuring the safety and efficacy of generic pharmaceutical products for human use. It plays a key role in the drug development period for both new drug products and their generic equivalents. These studies are also important in the post approval period in the presence of certain manufacturing changes. Information in the overall set of data that ensure the availability of safe and effective medicines to patients and practitioners can be discussed.
Related Conferences: Pharmaceutical Conferences | Biopharmaceutical Events | Pharma Conferences | Pharmaceutical Meetings | Biopharma Congress
8th Global Summit on Microbiology & Infectious Diseases, February 22-23, 2018 Paris, France; 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology, April 09-11, 2018 Amsterdam, Netherlands; 4th International Conference on Polymer Chemistry, June 25-27, 2018 Stockholm, Sweden; 11th World Microbiology & Immunology Congress, June 28-29, 2018 Amsterdam, Netherlands; 10th International Conference on Chemistry Education, June 21-22, 2018 Oslo, Norway;12th World Congress on Biotechnology and Microbiology, June 28-29, 2018 Amsterdam, Netherlands;14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, 10th World Congress on Neuropharmacology, August 28-30, 2018 Paris, France; Sweden; 8th World Congress on Chromatography, September 13-14, 2018 Prague, Czech Republic; 9th World Congress on Green Chemistry and Technology, September 17-19, 2018 Amsterdam, Netherlands; 17th Annual Congress on Pharmaceutics & Drug Delivery Systems, September 20-22, 2018 Prague, Czech Republic
Related Societies:
Association of the British Pharmaceutical Industry (ABPI), International Association for Pharmaceutical Technology(APV), National Pharmacy Association (NPA), Dutch Society for Clinical Pharmacology & Biopharmacy, European Association of Clinical Pharmacology and Therapeutics (EACPT), The International Society for Biopharmaceutical Statistics (ISBS), Chinese American BioPharmaceutical Society
Track 6: Clinical Trials on Biopharmaceuticals Products
Clinical Trials is a multidisciplinary program with broad participation with members from around the globe focused on learning about clinical research and its advances. This is your best opportunity to reach the largest assemblage of participants from Clinical Trials community that is from academia, clinical research entities, medical groups, related associations, societies and also from government agencies, pharmaceutical, biomedical and medical device industries.
Clinical Trials will discuss various disciplines involved in the pre-clinical research, conduct of clinical trials; it will educate health care researchers about design, operation, organizing, research computing, regulatory aspects and reporting of clinical trials. It promotes better understanding by the general public about the importance of clinical trials in prevention, diagnosis and treatment of disease.
Related Conferences: Pharmaceutical Conferences | Biopharmaceutical Events | Pharma Conferences | Pharmaceutical Meetings | Biopharma Congress
8th Global Summit on Microbiology & Infectious Diseases, February 22-23, 2018 Paris, France; 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology, April 09-11, 2018 Amsterdam, Netherlands; 4th International Conference on Polymer Chemistry, June 25-27, 2018 Stockholm, Sweden; 11th World Microbiology & Immunology Congress, June 28-29, 2018 Amsterdam, Netherlands; 10th International Conference on Chemistry Education, June 21-22, 2018 Oslo, Norway;12th World Congress on Biotechnology and Microbiology, June 28-29, 2018 Amsterdam, Netherlands;14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, 10th World Congress on Neuropharmacology, August 28-30, 2018 Paris, France; Sweden; 8th World Congress on Chromatography, September 13-14, 2018 Prague, Czech Republic; 9th World Congress on Green Chemistry and Technology, September 17-19, 2018 Amsterdam, Netherlands; 17th Annual Congress on Pharmaceutics & Drug Delivery Systems, September 20-22, 2018 Prague, Czech Republic
Related Associations or Societies: Generic Pharmaceutical Association | US Food and Drug Administration( FDA) | European Generic medicines Association | Therapeutics Goods Administration (TGA) | European Economic Area | Canadian Generic Pharmaceutical Association (CGPA) | Bioequivalence and Bioavailability forum | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry (AACC) | American Association of Pharmaceutical Scientists (AAPS) | Clinical Trials Information from National Institutes for Health (NIH) | National Institute of Standards and Technology (NIST)
Track 7: Pharmacovigilance
Pharmacovigilance is to provide a complete information reagarding drug safety and various benefits associated with them. Pharmacovigilance can help in providing information of unintended and severe adverse events which could not be provided by clinical trials involving in-vivo method. Pharmacovigilance legislation gives an outlook on the rules and regulations to follow in Pharmacovigilance practice. A Pharma industry in the improvement of pharmacovigilance system is very crucial to maintain the safety data, Detection and Evaluation of drug safety signals through manual and medical devices reporting. Pharmacovigilance scope also deals as Ecopharmacovigilance (EPV), pharmaco environmentology and pharmacovigilance in herbal medicines. Drug Safety is the pharmacological science relating to the collection, detection, assessment, monitoring, and prevention of adverse side effects with pharmacological action of pharmaceutical products. According to US FDA a drug is regarded as safe by looking at side effects, its manufacturing process and results of animal testing and clinical trials.
Related Conferences: Pharmaceutical Conferences | Biopharmaceutical Events | Pharma Conferences | Pharmaceutical Meetings | Biopharma Congress
8th Global Summit on Microbiology & Infectious Diseases, February 22-23, 2018 Paris, France; 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology, April 09-11, 2018 Amsterdam, Netherlands; 4th International Conference on Polymer Chemistry, June 25-27, 2018 Stockholm, Sweden; 11th World Microbiology & Immunology Congress, June 28-29, 2018 Amsterdam, Netherlands; 10th International Conference on Chemistry Education, June 21-22, 2018 Oslo, Norway;12th World Congress on Biotechnology and Microbiology, June 28-29, 2018 Amsterdam, Netherlands;14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, 10th World Congress on Neuropharmacology, August 28-30, 2018 Paris, France; Sweden; 8th World Congress on Chromatography, September 13-14, 2018 Prague, Czech Republic; 9th World Congress on Green Chemistry and Technology, September 17-19, 2018 Amsterdam, Netherlands; 17th Annual Congress on Pharmaceutics & Drug Delivery Systems, September 20-22, 2018 Prague, Czech Republic
Related Societies:
Association of the British Pharmaceutical Industry (ABPI), International Association for Pharmaceutical Technology(APV), National Pharmacy Association (NPA), Dutch Society for Clinical Pharmacology & Biopharmacy, European Association of Clinical Pharmacology and Therapeutics (EACPT), The International Society for Biopharmaceutical Statistics (ISBS), Chinese American BioPharmaceutical Society
Track 8: Drug Delivery System
Drug Delivery system attains greater global significance as Drug Delivery System plays a significant role in the future of pharmaceutical research Novel drug delivery system method by which a drug is delivered can have a significant effect on its efficacy. This includes topics like lipid Polymers to enhance drug delivery technology by providing controlled release of therapeutic agents in constant doses over long periods, cyclic dosage, and tunable release of both hydrophilic and hydrophobic drugs. The major part is to deliver an innovative speech on the latest Targeted drug delivery is a method of delivering medication to a patient in a manner that increases the concentration of the medication in some parts of the body relative to other drugs.
Related Conferences: Pharmaceutical Conferences | Biopharmaceutical Events | Pharma Conferences | Pharmaceutical Meetings | Biopharma Congress
8th Global Summit on Microbiology & Infectious Diseases, February 22-23, 2018 Paris, France; 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology, April 09-11, 2018 Amsterdam, Netherlands; 4th International Conference on Polymer Chemistry, June 25-27, 2018 Stockholm, Sweden; 11th World Microbiology & Immunology Congress, June 28-29, 2018 Amsterdam, Netherlands; 10th International Conference on Chemistry Education, June 21-22, 2018 Oslo, Norway;12th World Congress on Biotechnology and Microbiology, June 28-29, 2018 Amsterdam, Netherlands;14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, 10th World Congress on Neuropharmacology, August 28-30, 2018 Paris, France; Sweden; 8th World Congress on Chromatography, September 13-14, 2018 Prague, Czech Republic; 9th World Congress on Green Chemistry and Technology, September 17-19, 2018 Amsterdam, Netherlands; 17th Annual Congress on Pharmaceutics & Drug Delivery Systems, September 20-22, 2018 Prague, Czech Republic
Related Societies:
Accreditation Council for Pharmacy Education (ACPE), Association of British Healthcare Industries (ABHI), Association of the British Pharmaceutical Industry (ABPI), National Pharmacy Association (NPA), Dutch Society for Clinical Pharmacology & Biopharmacy, European Association of Clinical Pharmacology and Therapeutics (EACPT)
Track 9: Drug Disposition & Metabolism
Once a particular medicine is chosen, the principles of clinical pharmacokinetics are required to ensure the appropriate formulation of drug is chosen for an appropriate route of administration. On the basis of the patient’s drug handling parameters, which require an understanding of absorption, distribution, metabolism and excretion, the dosage regimen for the medicine in a particular patient can be developed. The pharmacist will then need to ensure that the appropriate regimen is prescribed to achieve optimal efficacy and minimal toxicity. Pharmacists then ensure that the appropriate monitoring is undertaken and that the patient receives the appropriate information to ensure compliance. Clinical pharmacokinetics is thus a fundamental knowledge base that pharmacists require to ensure effective practice of pharmaceutical care.
Related Conferences: Pharmaceutical Conferences | Biopharmaceutical Events | Pharma Conferences | Pharmaceutical Meetings | Biopharma Congress
8th Global Summit on Microbiology & Infectious Diseases, February 22-23, 2018 Paris, France; 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology, April 09-11, 2018 Amsterdam, Netherlands; 4th International Conference on Polymer Chemistry, June 25-27, 2018 Stockholm, Sweden; 11th World Microbiology & Immunology Congress, June 28-29, 2018 Amsterdam, Netherlands; 10th International Conference on Chemistry Education, June 21-22, 2018 Oslo, Norway;12th World Congress on Biotechnology and Microbiology, June 28-29, 2018 Amsterdam, Netherlands;14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, 10th World Congress on Neuropharmacology, August 28-30, 2018 Paris, France; Sweden; 8th World Congress on Chromatography, September 13-14, 2018 Prague, Czech Republic; 9th World Congress on Green Chemistry and Technology, September 17-19, 2018 Amsterdam, Netherlands; 17th Annual Congress on Pharmaceutics & Drug Delivery Systems, September 20-22, 2018 Prague, Czech Republic
Related Societies:
Association of the British Pharmaceutical Industry (ABPI), International Association for Pharmaceutical Technology(APV), National Pharmacy Association (NPA), Dutch Society for Clinical Pharmacology & Biopharmacy, European Association of Clinical Pharmacology and Therapeutics (EACPT), The International Society for Biopharmaceutical Statistics (ISBS), Chinese American BioPharmaceutical Society
Track 10: Biopharmaceutical Companies & Market analysis
Pharmaceutical analytic market research deals with the collection, analysis, and interpretation of details and information relating to the market environment of a given pharmaceutical product – in general of a medical drug. The primary objective of pharmaceutical market research is to gain as realistic and objective as possible an impression of the marketing opportunities of a given pharmaceutical product, thus enabling the identification of the chances and risks associated with its development potential as early on as possible.
Related Conferences: Pharmaceutical Conferences | Biopharmaceutical Events | Pharma Conferences | Pharmaceutical Meetings | Biopharma Congress
8th Global Summit on Microbiology & Infectious Diseases, February 22-23, 2018 Paris, France; 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology, April 09-11, 2018 Amsterdam, Netherlands; 4th International Conference on Polymer Chemistry, June 25-27, 2018 Stockholm, Sweden; 11th World Microbiology & Immunology Congress, June 28-29, 2018 Amsterdam, Netherlands; 10th International Conference on Chemistry Education, June 21-22, 2018 Oslo, Norway;12th World Congress on Biotechnology and Microbiology, June 28-29, 2018 Amsterdam, Netherlands;14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, 10th World Congress on Neuropharmacology, August 28-30, 2018 Paris, France; Sweden; 8th World Congress on Chromatography, September 13-14, 2018 Prague, Czech Republic; 9th World Congress on Green Chemistry and Technology, September 17-19, 2018 Amsterdam, Netherlands; 17th Annual Congress on Pharmaceutics & Drug Delivery Systems, September 20-22, 2018 Prague, Czech Republic
Related Societies:
Accreditation Council for Pharmacy Education (ACPE), Association of British Healthcare Industries (ABHI), Association of the British Pharmaceutical Industry (ABPI), National Pharmacy Association (NPA), Dutch Society for Clinical Pharmacology & Biopharmacy, European Association of Clinical Pharmacology and Therapeutics (EACPT)
Track 11: Pharmaceutical Regulatory Affairs and IPR of Biopharmaceuticals
Good Manufacturing Practices quality of drugs is essentially the responsibility of manufacturers. GMP Guidelines are means to assure this very quality of drugs. CGMP refers to the Current Good Manufacturing Practice regulations enforced by the US Food and Drug Administration (FDA). CGMPs provide for systems that assure proper design, monitoring, and control of manufacturing processes and facilities. Adherence to the CGMP regulations assures the identity, strength, quality, and purity of drug products by requiring that manufacturers of medications adequately control manufacturing operations. GMP is actually good common sense quality management quality assurance GMP production and quality control.
Related Conferences: Pharmaceutical Conferences | Biopharmaceutical Events | Pharma Conferences | Pharmaceutical Meetings | Biopharma Congress
8th Global Summit on Microbiology & Infectious Diseases, February 22-23, 2018 Paris, France; 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology, April 09-11, 2018 Amsterdam, Netherlands; 4th International Conference on Polymer Chemistry, June 25-27, 2018 Stockholm, Sweden; 11th World Microbiology & Immunology Congress, June 28-29, 2018 Amsterdam, Netherlands; 10th International Conference on Chemistry Education, June 21-22, 2018 Oslo, Norway;12th World Congress on Biotechnology and Microbiology, June 28-29, 2018 Amsterdam, Netherlands;14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, 10th World Congress on Neuropharmacology, August 28-30, 2018 Paris, France; Sweden; 8th World Congress on Chromatography, September 13-14, 2018 Prague, Czech Republic; 9th World Congress on Green Chemistry and Technology, September 17-19, 2018 Amsterdam, Netherlands; 17th Annual Congress on Pharmaceutics & Drug Delivery Systems, September 20-22, 2018 Prague, Czech Republic
Related Societies:
Pharmaceutical Society of Ireland, The International Society for Pharmaceutical Engineering (ISPE), European Association of Employed Community Pharmacists in Europe (EPhEU), Pharmaceutical Group of the European Union (PGEU).
Track 12: Biopharmaceutical Pharmacovigilance
The field of Pharmacovigilance is growing rapidly and its development is making tremendous impacts in medical sciences and pharmaceuticals. Euro Biopharma 2017 emphasizes on how the importance and significance can be gauged by the fact that it has made huge advancements over the course of time and is continuing to influence various sectors. With members from around the world focused on learning about Pharmacovigilance and its advances;
Related Conferences: Pharmaceutical Conferences | Biopharmaceutical Events | Pharma Conferences | Pharmaceutical Meetings | Biopharma Congress
8th Global Summit on Microbiology & Infectious Diseases, February 22-23, 2018 Paris, France; 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology, April 09-11, 2018 Amsterdam, Netherlands; 4th International Conference on Polymer Chemistry, June 25-27, 2018 Stockholm, Sweden; 11th World Microbiology & Immunology Congress, June 28-29, 2018 Amsterdam, Netherlands; 10th International Conference on Chemistry Education, June 21-22, 2018 Oslo, Norway;12th World Congress on Biotechnology and Microbiology, June 28-29, 2018 Amsterdam, Netherlands;14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, 10th World Congress on Neuropharmacology, August 28-30, 2018 Paris, France; Sweden; 8th World Congress on Chromatography, September 13-14, 2018 Prague, Czech Republic; 9th World Congress on Green Chemistry and Technology, September 17-19, 2018 Amsterdam, Netherlands; 17th Annual Congress on Pharmaceutics & Drug Delivery Systems, September 20-22, 2018 Prague, Czech Republic
Related Societies:
Accreditation Council for Pharmacy Education (ACPE), Association of British Healthcare Industries (ABHI), Association of the British Pharmaceutical Industry (ABPI), National Pharmacy Association (NPA), Dutch Society for Clinical Pharmacology & Biopharmacy, European Association of Clinical Pharmacology and Therapeutics (EACPT)
Track 13: Biotechnological Products
Pharmaceutical Biotechnology is an increasingly important area of science and technology, and contributes to design and delivery of new therapeutic drugs, the development of diagnostic agents for medical tests, and the beginnings of gene therapy for correcting the medical symptoms of hereditary diseases. Pharmaceutical Biotechnology is pioneering in the UK, providing a detailed insight into the technologies that allow the development and production of biopharmaceuticals that could lead to cures for most major diseases.
Related Conferences: Pharmaceutical Conferences | Biopharmaceutical Events | Pharma Conferences | Pharmaceutical Meetings | Biopharma Congress
8th Global Summit on Microbiology & Infectious Diseases, February 22-23, 2018 Paris, France; 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology, April 09-11, 2018 Amsterdam, Netherlands; 4th International Conference on Polymer Chemistry, June 25-27, 2018 Stockholm, Sweden; 11th World Microbiology & Immunology Congress, June 28-29, 2018 Amsterdam, Netherlands; 10th International Conference on Chemistry Education, June 21-22, 2018 Oslo, Norway;12th World Congress on Biotechnology and Microbiology, June 28-29, 2018 Amsterdam, Netherlands;14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, 10th World Congress on Neuropharmacology, August 28-30, 2018 Paris, France; Sweden; 8th World Congress on Chromatography, September 13-14, 2018 Prague, Czech Republic; 9th World Congress on Green Chemistry and Technology, September 17-19, 2018 Amsterdam, Netherlands; 17th Annual Congress on Pharmaceutics & Drug Delivery Systems, September 20-22, 2018 Prague, Czech Republic
Related Societies:
Association of the British Pharmaceutical Industry (ABPI), International Association for Pharmaceutical Technology(APV), National Pharmacy Association (NPA), Dutch Society for Clinical Pharmacology & Biopharmacy, European Association of Clinical Pharmacology and Therapeutics (EACPT), The International Society for Biopharmaceutical Statistics (ISBS), Chinese American BioPharmaceutical Society
Track 14: Drug Delivery from Plant Extracts
Nanotechnology has been increasingly employed in drug delivery as it increases the drug dissolution rate, leading to enhanced drug absorption and bioavailability. Many nanostructures have been proposed for drug delivery, each one having their own advantages and drawbacks. This manuscript revises the use of lipid- and polymer-based nanostructures in the association of different extract, establishing a relation between the type of Nano systems and its preparation method to the different plant extracts and most abundant compounds. Depending on the method of extraction, plant extracts can contain an enormous variety of active molecules, such as phenolic compounds, essential oils, alkaloids, among others. In many cases, from a pharmacological point of view, it is interesting to work with crude extract or fractions instead of a single isolated compound. In any case, in order to achieve a final product some issues must be overcome, including poor stability, solvent toxicity, and low solubility of the bioactive compound.
Related Conferences: Pharmaceutical Conferences | Biopharmaceutical Events | Pharma Conferences | Pharmaceutical Meetings | Biopharma Congress
8th Global Summit on Microbiology & Infectious Diseases, February 22-23, 2018 Paris, France; 14th International Conference and Exhibition on Nanomedicine and Pharmaceutical Nanotechnology, April 09-11, 2018 Amsterdam, Netherlands; 4th International Conference on Polymer Chemistry, June 25-27, 2018 Stockholm, Sweden; 11th World Microbiology & Immunology Congress, June 28-29, 2018 Amsterdam, Netherlands; 10th International Conference on Chemistry Education, June 21-22, 2018 Oslo, Norway;12th World Congress on Biotechnology and Microbiology, June 28-29, 2018 Amsterdam, Netherlands;14th World Congress on Pharmacology and Drug Safety, August 15-17, 2018 Stockholm, 10th World Congress on Neuropharmacology, August 28-30, 2018 Paris, France; Sweden; 8th World Congress on Chromatography, September 13-14, 2018 Prague, Czech Republic; 9th World Congress on Green Chemistry and Technology, September 17-19, 2018 Amsterdam, Netherlands; 17th Annual Congress on Pharmaceutics & Drug Delivery Systems, September 20-22, 2018 Prague, Czech Republic
Related Associations or Societies: Generic Pharmaceutical Association | US Food and Drug Administration( FDA) | European Generic medicines Association | Therapeutics Goods Administration (TGA) | European Economic Area | Canadian Generic Pharmaceutical Association (CGPA) | Bioequivalence and Bioavailability forum | FDA Office of Surveillance and Epidemiology | American Association for Clinical Chemistry (AACC) | American Association of Pharmaceutical Scientists (AAPS) | Clinical Trials Information from National Institutes for Health (NIH) | National Institute of Standards and Technology (NIST)
The opportunity in biopharmaceuticals is big and growing too rapidly to ignore. Today, biopharmaceuticals generate global revenues of $163 billion, making up about 20%of the pharma market. It’s by far the fastest-growing part of the industry: biopharma’s current annual growth rate of more than 8% is double that of conventional pharma, and growth is expected to continue at that rate for the foreseeable future.
The future of Biopharmaceutical industry is promising with biotech companies focusing more on innovation & technological advancements & increasing interest of pharmaceutical companies to enter into biotech business.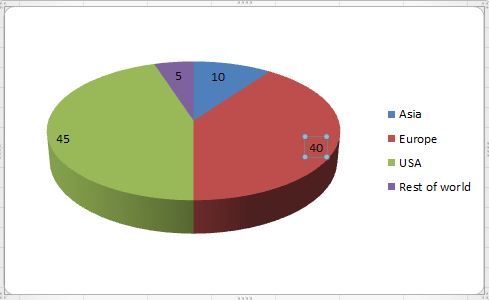
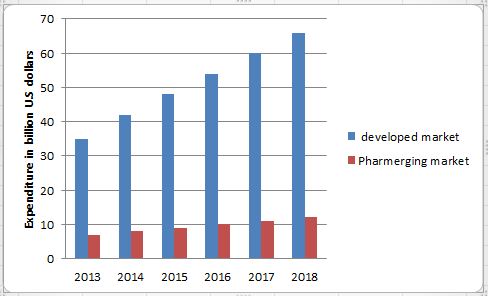
Industry average. The current biologics-development pipeline supports an outlook of continued healthy growth. The number of biotech patents applied Investing in biotech R&D has yielded better returns than the pharma-for every year has been growing at 25% annually since 2005. There are currently more than 1,500 biomolecules undergoing clinical trials, and the success rate for biologics has so far been over twice that of small-molecule products, with 13%of biopharma products that enter the Phase I trial stage going on to launch.
The success of the clinical pipeline will lead to an unprecedented number of new molecule launches, rising from a handful a few years ago to 10 to 15 annually, as biopharma products make up an increasing share of new approvals from the US Food and Drug Administration in the future. A further steep increase is to be expected as multiple players begin to receive approval for the production of biosimilars after 2015.
Past Conference Report
Euro Biopharma 2017
We gratefully thank all our wonderful Keynote Speakers, Speakers, Conference Attendees, Students, Organizing Committee Members, Associations, Sponsors, Exhibitors and Media Partners for making Euro Biopharma 2017 Conference the best ever!
The 4thEuropean Biopharma Congress, hosted by the Conference series LLC was held during November 9-11, 2017 at Vienna, Austria. Benevolent response and active participation was received from the Organizing Committee Members along with Scientists, Researchers, Students and leaders from various fields of Clinical Research & Trials, clinical research entities, medical groups, related associations, societies and also from government agencies, pharmaceutical, biomedical and medical device industries.
Conference series LLC expresses its gratitude to the conference Moderators, OCM’S, Keynotes Session, Chairs for taking up the responsibility to coordinate during the sessions. We are indebted to your support.
The meeting reflected various sessions, in which discussions were held by the following eminent speakers:
· Vladimir P Torchilin, Northeastern University, USA
· Hiroshi Ohrui, Yokohama University of Pharmacy, Japan
· Wil fried Dimpfel, University of Giessen, Germany
· Jamal Ouazzani, National Center for Scientific Research CNRS, France
· Amots Dafni, University Haifa, Israel
· Roger RB Leakey, International Tree Foundation, UK
· Halina Baran, Karl Landsteiner Research Institute for Neurochemistry and Neuropharmacology,
Austria
· Geert C Mudde, OncoQR ML GmbH, Austria
· Kang Choon Lee, Sungkyunkwan University, South Korea
· Anupam Bishayee, Larkin University, USA
A very special Thanks to our Media Partners and Exhibitors to have bestowed and their faith in collaborating with us to make this event a fruitful one. We hope you continue your support in our future endeavors.
With the grand success of Euro Biopharma 2017, Conference Series is proud to announce the “6th European Biopharma Congress ” September 18-19, 2018 at Amsterdam, Netherlands
Let us meet again
@
Conference Highlights
- Biopharmaceuticals
- Biopharmaceutics: Drug Discovery and Development
- Therapeutic Biological Products
- Biologics and Biosimilars
- clinical Trials on Biopharmaceutical Products
- Drug Delivery System of Biopharmaceutical Products
- Cellular and Gene Therapy
- Bioavailability/Bioequivalence on Biopharmaceutical Products
- Biotechnological Products
- Drug Disposition & Pharmacokinetics
- Pharmaceutical Regulatory Affairs And IPR
- Biopharmaceutical Pharmacovigilance
- Drug Dissolution of Biopharmaceutical Products
- Biopharmaceutical Companies & Market Analysis
- Entrepreneurs Investment Meet
- Pharmacovigilance
- Drug Delivery from Plant Extracts
To share your views and research, please click here to register for the Conference.
To Collaborate Scientific Professionals around the World
| Conference Date | September 18-19, 2018 | ||
| Sponsors & Exhibitors |
|
||
| Speaker Opportunity Closed | Day 1 | ||
| Poster Opportunity Closed | Click Here to View | ||
Useful Links
Special Issues
All accepted abstracts will be published in respective Our International Journals.
- Pharmaceutics & Novel Drug Delivery System
- Drug Discovery & Designing
- Pharmacovigilance & Drug Safety
Abstracts will be provided with Digital Object Identifier by










